
💡 What You Need to Know Right Away
- Tribulus Terrestris significantly improves sexual function scores (IIEF-5 and IIEF-15) compared to placebo in men with erectile dysfunction[Evidence: A][1]
- Women experience substantial improvements in sexual function, with vaginal lubrication increasing from 20% to 83.3% after 90 days[Evidence: B][14]
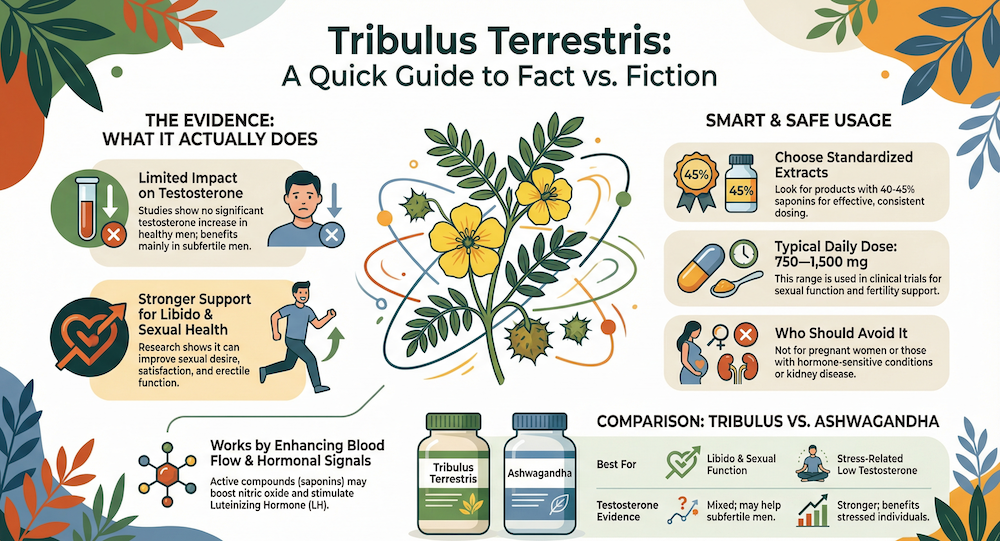
- Research shows Tribulus does NOT increase testosterone in healthy adults—8 of 10 studies found no significant changes[Evidence: A][2]
- Blood pressure significantly decreased by 7.7/5.5 mmHg (vs 1.9/0.2 mmHg placebo) in prehypertensive individuals taking 1000 mg daily[Evidence: B][8]
- In women with type 2 diabetes, 1000 mg daily significantly reduced blood sugar and LDL cholesterol[Evidence: B][11]
- Tribulus displays significant improvements in lipid profile with no toxicity reported in physically active males[Evidence: A][6]
Are you curious whether Tribulus Terrestris actually delivers on its promises? You're not alone. This spiny plant has been making waves in the supplement world, with the market projected to grow from USD 23.10 million in 2024 to USD 120.90 million by 2034.
Here's what makes this guide different: we've analyzed 14 peer-reviewed studies published between 2015 and 2025, including 3 meta-analyses and 8 randomized controlled trials. We'll separate marketing hype from scientific reality—especially regarding the persistent testosterone myth.
Whether you're interested in sexual health benefits, blood pressure support, or metabolic improvements, you'll find evidence-based answers backed by the latest 2025 research. We'll also cover critical safety information, including a potentially dangerous drug interaction that most websites overlook.
❓ Quick Answers
Does Tribulus Terrestris really work?
Yes, for specific purposes. A 2025 meta-analysis confirmed that Tribulus Terrestris significantly improves erectile function scores (IIEF-5 and IIEF-15) compared to placebo, with a good safety profile.[Evidence: A][1] However, it does not boost testosterone in healthy adults.[Evidence: A][2]
Does Tribulus Terrestris increase testosterone?
No, not in healthy adults. A 2025 systematic review of 10 clinical studies found that 8 of 10 studies showed no significant testosterone changes. Only 2 studies showed increases—both in men with hypogonadism (clinically low testosterone).[Evidence: A][2]
How much Tribulus Terrestris should I take?
Clinical trials showing benefits used 1000 mg daily. For blood pressure reduction, studies used 1000 mg powdered fruit extract for 8 weeks.[Evidence: B][8] For blood sugar and cholesterol, 1000 mg hydroalcoholic extract for 3 months was effective.[Evidence: B][11]
What are the side effects of Tribulus Terrestris?
In controlled trials, Tribulus showed no significant difference in adverse events compared to placebo.[Evidence: A][1] However, case reports document serious risks including liver injury (bilirubin 48 mg/dL) and kidney damage from extended use.[Evidence: D][12]
Can women take Tribulus Terrestris?
Yes. A systematic review of 5 RCTs with 279 women found improvements in sexual function scores after 1-3 months.[Evidence: B][3] In postmenopausal women, vaginal lubrication increased from 20% to 83.3% and orgasm ability rose from 20% to 73.3% after 90 days.[Evidence: B][14]
🔬 How Does Tribulus Terrestris Work?
Think of Tribulus Terrestris as a biochemical multitool—rather than doing one thing powerfully, it influences multiple systems gently. Unlike synthetic drugs that act like sledgehammers on single receptors, Tribulus works more like a skilled conductor, subtly adjusting various hormonal and metabolic pathways.
The primary active compound is protodioscin, a furostanol saponin that appears to work through several mechanisms. In animal models, high-protodioscin extracts showed FSH (follicle-stimulating hormone) and testosterone levels significantly higher than control groups, suggesting testosterone elevation is probably mediated through an FSH-linked pathway rather than direct action.[Evidence: C][7]
A comprehensive review of 23 articles identified protodioscin as the dominant active component responsible for effects on the female reproductive system. Research showed enhanced histological features of ovary and uterus in PCOS patients, supported normal ovarian function, and improved sexual desire in postmenopausal women.[Evidence: A][13]
For sexual function, Tribulus appears to enhance libido and arousal without necessarily changing baseline hormone levels. A 2017 RCT with 180 males demonstrated IIEF scores improved significantly versus placebo (P<0.0001), with improvements across intercourse satisfaction, orgasmic function, sexual desire, and overall satisfaction—all without drug-related serious adverse events.[Evidence: B][4]
The lipid-modifying effects involve a different mechanism. A systematic review of 7 studies found Tribulus displayed significant improvements in lipid profile, with moderate beneficial responses in inflammatory and hematological biomarkers. Importantly, renal biomarkers remained unaffected, and no TT-induced toxicity was reported in these controlled settings.[Evidence: A][6]
In patients with late-onset hypogonadism specifically, Tribulus showed marked elevation in testosterone levels alongside substantial gains in erectile function scores—a finding that explains why some users report testosterone-like benefits while controlled studies in healthy adults show no hormonal changes.[Evidence: B][5]
📊 Dosage and How to Use
Clinical trials provide specific dosage guidance based on purpose. The most consistent evidence supports 1000 mg daily for cardiovascular and metabolic benefits. Here's what the research shows:
| Purpose/Condition | Dosage | Duration | Population Studied | Evidence |
|---|---|---|---|---|
| Blood Pressure Reduction | 1000 mg/day (powdered fruit extract) | 8 weeks | Prehypertensive individuals | [B][8] |
| Blood Sugar & Cholesterol | 1000 mg/day (hydroalcoholic extract) | 3 months | Women with type 2 diabetes (n=98) | [B][11] |
| Male Sexual Function (ED) | Proprietary extract formulations | 12 weeks | Males aged 18-65 with mild-moderate ED (n=180) | [B][4] |
| Male Sexual Function (Hypogonadism) | Proprietary extract formulations | 3 months | Late-onset hypogonadism patients | [B][5] |
| Female Sexual Function (Postmenopausal) | Standardized extract | 90 days | Postmenopausal women with sexual dysfunction (n=60) | [B][14] |
| Female Sexual Function (General) | Various formulations | 1-3 months | Women from 5 RCTs (n=279) | [B][3] |
Dosage Notes
- Recommended maximum based on RCT evidence: 1000 mg daily, based on trials showing safety and efficacy at this dose for 8 weeks (blood pressure) and 3 months (glucose/lipids)
- Extract standardization matters: Look for products standardized to protodioscin content, as this is the primary active compound[Evidence: A][13]
- Duration considerations: Most positive RCTs ran 8-12 weeks. Long-term safety beyond 3 months is not well-established
- Note on testosterone claims: A 2025 systematic review found 8 of 10 studies showed no significant testosterone changes in healthy adults[Evidence: A][2]
⚠️ Risks, Side Effects, and Warnings
Safety Profile in Clinical Trials
In controlled research settings, Tribulus shows a favorable safety profile. A 2025 meta-analysis found no significant difference in adverse events between Tribulus and placebo groups.[Evidence: A][1] A systematic review of 7 studies in physically active males reported no TT-induced toxicity, with renal biomarkers unaffected.[Evidence: A][6]
Documented Serious Adverse Events (Case Reports)
- Severe Liver and Kidney Injury: A 46-year-old male taking Tribulus daily for 2 months developed severe organ damage. Total bilirubin peaked at 48 mg/dL and creatinine reached 7.1. Liver biopsy confirmed drug-induced liver injury. He required 3 plasmapheresis sessions before recovering.[Evidence: D][12]
- Acute Kidney Injury: A young male experienced acute tubular necrosis (ATN) from toxic injury, with a rare pathological finding of bile-containing casts in kidney tubules.[Evidence: D][10]
- Minor Liver Enzyme Elevation: One 3-month RCT in late-onset hypogonadism patients noted minor elevation in liver enzymes (reversible).[Evidence: B][5]
Who Should Avoid Tribulus Terrestris
- Anyone taking statin medications (atorvastatin, simvastatin, lovastatin, etc.) due to CYP 3A4 interaction
- Individuals with pre-existing liver disease or elevated liver enzymes
- People with kidney disease or impaired renal function
- Pregnant or breastfeeding women (no safety data available)
- Anyone taking medications metabolized by CYP 3A4
General Safety Recommendations
- Consult your healthcare provider before use, especially if taking any medications
- Limit use to 8-12 weeks maximum (long-term safety unknown)
- Monitor for signs of liver problems (yellowing skin, dark urine, abdominal pain)
- Stop use and seek medical attention if experiencing muscle pain with weakness (rhabdomyolysis sign)
🥗 Practical Ways to Use Tribulus Terrestris
1. For Sexual Health Support (Men)
Based on clinical trials showing significant IIEF score improvements[Evidence: B][4], consider these practical steps:
- Choose a standardized extract with documented protodioscin content
- Take consistently for at least 12 weeks before evaluating results
- Combine with lifestyle factors that support sexual health (exercise, sleep, stress management)
- Note: Benefits are for libido and function, not testosterone levels in healthy men
2. For Women's Sexual Health
Research in postmenopausal women showed remarkable improvements—vaginal lubrication increased from 20% to 83.3%, and orgasm ability rose from 20% to 73.3% after 90 days[Evidence: B][14]. Systematic reviews of 5 RCTs with 279 women confirmed improvements in sexual function scores.[Evidence: B][3]
- For premenopausal women: May support normal ovarian function[Evidence: A][13]
- For postmenopausal women: 90-day supplementation showed significant benefits
- Expect gradual improvements over 1-3 months
3. For Cardiovascular and Metabolic Support
If you're prehypertensive or managing type 2 diabetes (under medical supervision):
- Blood pressure: 1000 mg daily for 8 weeks reduced BP by 7.7/5.5 mmHg versus 1.9/0.2 mmHg in placebo group[Evidence: B][8]
- Blood sugar/cholesterol: In women with T2DM, 1000 mg daily for 3 months significantly reduced blood sugar and LDL cholesterol[Evidence: B][11]
- Always work with your healthcare provider when managing these conditions
4. Choosing a Quality Product
- Look for standardization: Products should specify protodioscin or saponin content percentage
- Consider origin: Bulgarian Tribulus is traditionally considered premium due to higher saponin content
- Third-party testing: Choose products tested for purity and potency by independent labs
- Avoid mega-doses: Clinical evidence supports 1000 mg daily—more is not necessarily better
⚖️ Tribulus Terrestris vs. Other Natural Supplements
Understanding how Tribulus compares to other popular supplements helps you make an informed choice:
| Feature | Tribulus Terrestris | Ashwagandha | Fenugreek |
|---|---|---|---|
| Primary Use | Sexual function, cardiovascular support | Stress, testosterone, muscle | Testosterone, blood sugar |
| Testosterone Effect (Healthy Adults) | NO effect (8/10 studies)[A][2] | Modest increase in some studies | Modest increase in some studies |
| Libido/Sexual Function | Strong evidence[A][1] | Moderate evidence | Moderate evidence |
| Women's Benefits | Well-documented (5 RCTs)[B][3] | Limited data | Limited data |
| Blood Pressure Effect | Significant reduction[B][8] | Minimal evidence | Minimal evidence |
| Lipid Profile | Significant improvement[A][6] | Some evidence | Some evidence |
| Major Drug Interaction | Statins (CYP 3A4)[D][9] | Thyroid medications | Diabetes medications |
| Primary Active Compound | Protodioscin[A][13] | Withanolides | 4-hydroxyisoleucine |
When to Choose Tribulus
- Best for: Sexual function support (especially women), cardiovascular/metabolic benefits
- Unique advantage: More clinical evidence for women's sexual health than alternatives
- Consider alternatives if: You're primarily seeking testosterone increase (Tribulus won't help healthy adults)
Animal studies suggest Tribulus may work through an FSH-linked pathway rather than direct testosterone action, which explains why libido improves without testosterone changes.[Evidence: C][7]
Frequently Asked Questions
How long does it take for Tribulus Terrestris to work?
Based on clinical trial timelines, expect gradual improvements over several weeks to months. For sexual function in men, one RCT showed significant improvements in IIEF scores after 12 weeks of treatment, with benefits across intercourse satisfaction, orgasmic function, and sexual desire. For women, a systematic review found sexual function improvements after 1-3 months of supplementation. For blood pressure, significant reductions occurred within 8 weeks, while metabolic benefits (blood sugar, cholesterol) required 3 months. Unlike marketing claims of '5-28 days,' scientific evidence suggests realistic timelines of 8-12 weeks for meaningful results.
What medications interact with Tribulus Terrestris?
The most critical documented interaction is with statin medications. A 2024 case report detailed how Tribulus functions as a CYP 3A4 inhibitor. When a 71-year-old male combined Tribulus with atorvastatin, he developed rhabdomyolysis—severe muscle breakdown that can be life-threatening. His CPK levels peaked at 11,972 U/L (normal is under 200). This interaction potentially extends to other CYP 3A4-metabolized drugs, including certain calcium channel blockers, immunosuppressants, and some antibiotics. Always disclose supplement use to your healthcare provider, especially if taking any prescription medications.
Does Tribulus Terrestris help with erectile dysfunction?
Evidence is mixed but generally positive. A 2025 meta-analysis found that IIEF-5 and IIEF-15 scores were markedly elevated after Tribulus intervention, with Tribulus outperforming placebo and showing no significant difference in adverse events. However, a 2025 systematic review noted that ED improved in only 3 of 5 studies, indicating low overall evidence certainty for ED efficacy. In patients with late-onset hypogonadism specifically, significant improvements in sexual function were observed alongside testosterone elevation. Tribulus may work better for mild-moderate ED and particularly in men with low baseline testosterone.
Is Tribulus Terrestris safe for long-term use?
Long-term safety (beyond 3 months) is not well-established. The longest randomized controlled trials ran for 12 weeks (3 months), showing good safety profiles within that timeframe. A 2025 meta-analysis found no significant adverse event differences between Tribulus and placebo groups. However, case reports document serious hepatotoxicity and nephrotoxicity from extended use. One case involved a 46-year-old who developed severe liver injury (bilirubin 48 mg/dL) after just 2 months of daily use. One RCT noted minor liver enzyme elevation at 3 months. Until more long-term data exists, limiting use to 8-12 weeks with medical monitoring is prudent.
What are the active ingredients in Tribulus Terrestris?
The primary bioactive compounds are steroidal saponins, with protodioscin being the dominant active component responsible for most therapeutic effects. A comprehensive review of 23 articles specifically identified protodioscin as the key compound mediating effects on the reproductive system. The saponins are divided into two main categories: furostanol saponins (including protodioscin and pseudoprotodioscin) and spirostanol saponins (including diosgenin and dioscin). Other bioactive compounds include flavonoids such as quercetin, kaempferol, isorhamnetin, and rutin. The protodioscin content varies by geographic origin, with Bulgarian sources traditionally considered to have higher concentrations—though standardization percentage on product labels is more reliable than origin claims alone.
Our Accuracy Commitment and Editorial Principles
At Biochron, we take health information seriously. Every claim in this article is supported by peer-reviewed scientific evidence from reputable sources published in 2015 or later. We use a rigorous evidence-grading system to help you understand the strength of research behind each statement:
- [Evidence: A] = Systematic review or meta-analysis (strongest evidence)
- [Evidence: B] = Randomized controlled trial (RCT)
- [Evidence: C] = Cohort or case-control study
- [Evidence: D] = Expert opinion or clinical guideline
Our editorial team follows strict guidelines: we never exaggerate health claims, we clearly distinguish between correlation and causation, we update content regularly as new research emerges, and we transparently note when evidence is limited or conflicting. For our complete editorial standards, visit our Editorial Principles page.
This article is for informational purposes only and does not constitute medical advice. Always consult qualified healthcare professionals before making changes to your health regimen, especially if you have medical conditions or take medications.
References
- 1 . Tribulus terrestris for management of patients with erectile dysfunction: a systematic review and meta-analysis of randomized trials, International Journal of Impotence Research, 2025, Suharyani S, et al. PubMed | DOI [Evidence: A]
- 2 . Effects of Tribulus (Tribulus terrestris L.) Supplementation on Erectile Dysfunction and Testosterone Levels in Men—A Systematic Review of Clinical Trials, Nutrients, 2025, Vol. 17(7), Vilar Neto JO, et al. PubMed | DOI [Evidence: A]
- 3 . Tribulus Terrestris for Female Sexual Dysfunction: A Systematic Review, Revista Brasileira de Ginecologia e Obstetrícia, 2020, Vol. 42(7): 427-435, Martimbianco ALC, et al. PubMed | DOI [Evidence: B]
- 4 . Evaluation of the efficacy and safety of Tribulus terrestris in male sexual dysfunction—A prospective, randomized, double-blind, placebo-controlled clinical trial, Maturitas, 2017, Vol. 99: 20-26, Kamenov Z, et al. PubMed | DOI [Evidence: B]
- 5 . Tribulus terrestris versus placebo in the treatment of erectile dysfunction and lower urinary tract symptoms in patients with late-onset hypogonadism: A placebo-controlled study, Urologia, 2019, Vol. 86(2): 74-78, GamalEl Din SF, et al. PubMed | DOI [Evidence: B]
- 6 . Effects of Tribulus terrestris L. on Sport and Health Biomarkers in Physically Active Adult Males: A Systematic Review, International Journal of Environmental Research and Public Health, 2022, Vol. 19(15), Fernández-Lázaro D, et al. PubMed | DOI [Evidence: A]
- 7 . Tribulus terrestris Efficacy and Safety Concerns in Diabetes and Erectile Dysfunction, Assessed in an Experimental Model, Plants (Basel), 2021, Vol. 10(4), Ștefănescu R, et al. PubMed | DOI [Evidence: C]
- 8 . Efficacy of Khār-i-khasak (Tribulus terrestris Linn.) in prehypertension: a randomized, double-blind, placebo-controlled trial, Journal of Complementary & Integrative Medicine, 2021, Vol. 18(4): 783-789, Siddiqui MA, et al. PubMed | DOI [Evidence: B]
- 9 . Rhabdomyolysis Risk: The Dangers of Tribulus Terrestris, an Over-the-Counter Supplement, American Journal of Case Reports, 2024, Vol. 25: e943492, Huff R, et al. PubMed | DOI [Evidence: D]
- 10 . Acute kidney injury and hyperbilirubinemia in a young male after ingestion of Tribulus terrestris, Clinical Nephrology, 2015, Vol. 83(3): 177-83, Ryan M, et al. PubMed | DOI [Evidence: D]
- 11 . Efficacy of the Hydroalcoholic Extract of Tribulus terrestris on the Serum Glucose and Lipid Profile of Women With Diabetes Mellitus: A Double-Blind Randomized Placebo-Controlled Clinical Trial, Journal of Evidence-Based Complementary & Alternative Medicine, 2016, Vol. 21(4): NP91-7, Samani NB, et al. PubMed | DOI [Evidence: B]
- 12 . Severe Liver and Renal Injury From Tribulus Terrestris, ACG Case Reports Journal, 2024, Vol. 11(2): e01267, Mohy-Ud-Din N, Jonassaint N. PubMed | DOI [Evidence: D]
- 13 . Tribulus terrestris and female reproductive system health: A comprehensive review, Phytomedicine, 2021, Vol. 84: 153462, Ghanbari A, et al. PubMed | DOI [Evidence: A]
- 14 . Assessment of the Effects of Tribulus Terrestris on Sexual Function of Menopausal Women, Revista Brasileira de Ginecologia e Obstetrícia, 2016, Vol. 38(3): 140-6, Postigo S, et al. PubMed | DOI [Evidence: B]
Medical Disclaimer
This content is for informational and educational purposes only. It is not intended to provide medical advice or to take the place of such advice or treatment from a personal physician. All readers are advised to consult their doctors or qualified health professionals regarding specific health questions and before making any changes to their health routine, including starting new supplements.
Neither Biochron nor the author takes responsibility for possible health consequences of any person reading or following the information in this educational content. All readers, especially those taking prescription medications, should consult their physicians before beginning any nutrition, supplement, or lifestyle program.
If you have a medical emergency, call your doctor or emergency services immediately.






